|
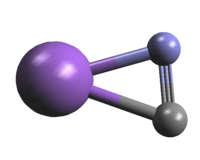
The molecule is nearly T-shaped with the NaN bond slightly
shorter than the NaC bond. Therefore, the molecule should be
considered more as sodium isocyanide rather than as
sodium cyanide.
The first entry from Apr. 2007 has been revised based on
an analysis from
(1) H. S. P. Müller, D. T. Halfen, and
L. M. Ziurys,
2012, J. Mol. Spectrosc. 272, 23.
The experimental measurements were taken
from
(2) J. J. van Vaals, W. L. Meerts, and A. Dymanus,
1984, Chem. Phys. 86, 147;
and from
(3) D. T. Halfen and L. M. Ziurys,
2011, Astrophys. J. 730, Art. No. 107.
As described in (1), the reported uncertainties in (2) were
assumed to be 3 σ values. Uncertainties for
the lower Ka transition frequencies from
(3) were probably judged somewhat conservatively.
On the other hand, three transition frequencies from (3)
were omitted fromthe final fit. The predicted transition
frequencies should be fairly reliable as long as their
uncertainties do not exceed 1 MHz.
While in the laboratory b-type transitions have been
observed, their intensities are likely very small because
the b dipole moment component is about 1.5 orders
of magnitude smaller than the a component; hence,
transitions will be about three orders of magnitude weaker.
Hyperfine structure splitting caused by the 14N nucleus
or the 23Na nucleus are not of relevance for
astronomical observations.
The ab initio dipole moment was taken
from
(4) H. S. P. Müller, 2007, unpublished.
Note: in the original detection paper, the dipole moment
from an ab initio paper was taken as being in units of Debye,
whereas it was given in atomic units; in Debye, the dipole moment
is larger by a factor of 2.542 !
|