Sodium cyanide, sodium isocyanide; 13C isotopic species
 | |
|---|---|
| Species tag | 050513 |
| Version | 2* |
| Date of Entry | Jan. 2012 |
| Contributor | H. S. P. Müller |
|
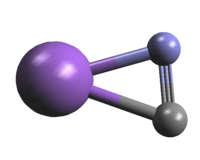
The molecule is nearly T-shaped with the NaN bond slightly
shorter than the NaC bond. Therefore, the molecule should be
considered more as sodium isocyanide rather than as
sodium cyanide. | |
| Lines Listed | 2706 |
| Frequency / GHz | < 1000 |
| Max. J | 71 |
| log STR0 | -6.0 |
| log STR1 | -4.0 |
| Isotope Corr. | -1.972 |
| Egy / (cm–1) | 0.0 |
| µa / D | 8.85 |
| µb / D | |
| µc / D | |
| A / MHz | 55674.35 |
| B / MHz | 8198.012 |
| C / MHz | 7106.818 |
| Q(300.0) | 15543.8862 |
| Q(225.0) | 10070.8918 |
| Q(150.0) | 5469.2970 |
| Q(75.00) | 1930.3029 |
| Q(37.50) | 682.6513 |
| Q(18.75) | 241.9388 |
| Q(9.375) | 86.0356 |
| Q(9.375) | 33.8674 |
| Q(9.375) | 13.8935 |
| detected in ISM/CSM | no |